When the FDA releases guidances in burst mode
By Mitch on Friday, 20 March 2015, 17:03 - Regulations - Permalink
If you watch from time to time the new guidances released by the FDA, you probably noticed that two guidances about software were released in january and february 2015.
We have the :
- Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices guidance released in February,
- General Wellness: Policy for Low Risk Devices Draft Guidance released in January,
- Medical Device Accessories: Defining Accessories and Classification Pathway for New Accessory Types Draft Guidance released in January.
Add to these very recent guidances, the :
- Mobile Medical Applications guidance released in september.
That's a lot of stuff to read. I'll try to sum up all of these.
About MDDS and PACS
The first guidance Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices, is actually about PACS servers and Health IT systems, without modules performing data processing for diagnostic or treatment purposes.
The guidance simply says that PACS servers and MDDS are actually medical devices, but the FDA doesn't intend to regulate them.
This produced a lot of fuss in the medical devices galaxy. But this isn't a scam or a joke. If you want to confirm by yourself that this is true,
- First, have a look at the recent FDA webinar Overview of Medical Device Data Systems, General Wellness Devices, and Medical Device Accessories - February 24, 2015, the question is raised by many attendants and the FDA speaker's answer is clear of this subject,
- Second, if you have a doubt, contact the FDA to confirm that your software falls in the scope of this guidance,
- Third, enjoy.
Anyway, if your system falls in the guidance scope today, this may not be the case tomorrow. If you plan to add advanced modules processing data for clinical purposes, you should anticipate this evolution:
- Either by insulating advanced modules and concealing the rest of the system in SOUP,
- Or, to be safe, by applying the IEC 62304 requirements to have the required documentation when your system is back to the medical device regulated zone.
About Mobile Apps
The two following guidances are about mobile apps:
- The Mobile Medical Applications guidance,
- The General Wellness draft guidance.
The slides of the FDA Webinar contain a simple diagram, which explains at a glance the status of mobile apps.
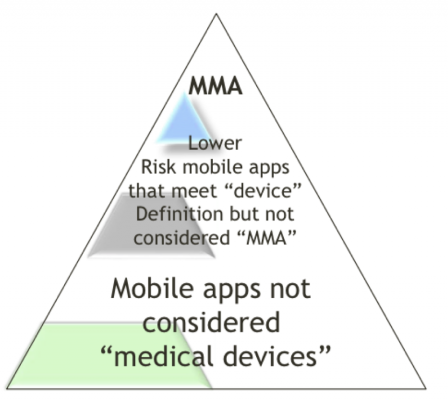 The triangle symbolizes the volumes of apps available on mobile apps stores: thousands at the bottom, a dozen at the top.
The triangle symbolizes the volumes of apps available on mobile apps stores: thousands at the bottom, a dozen at the top.
The triangle is split in three zones:
- Top: medical devices with no doubt on the intended use,
- Bottom: general wellness devices with no doubt on the non-medical intended use,
- Middle: the gray area where the intended use or the level of risks of the apps shall (yes, shall) be assessed with the FDA.
Thus, if you have doubts about the medical device status of your mobile app, contact the FDA. They will decide whether your device is regulated or not, based on its intended use and risks identified in the use of your device. This is what they call law enforcement discretion.
About accessories
The last guidance we haven't commented yet is the Medical Device Accessories draft guidance.
While this guidance is not focussed on software, it has consequences on software. Many mobile apps are used to remotely access a medical device or medical data. Thus, they could be qualified as accessories.
The guidance gives the definition of an accessory. If your app is an accessory, it is likely to be at the top of the triangle. Based on risk assessment, this accessory may come closer to the tip of the triangle or get closer to the grey zone.
For innovative mobile medical apps without predicates, the FDA encourages manufacturers and other parties (...) to utilize the de novo classification process. With this guidance, the FDA leaves the door open for a clearance process less painful than a PMA (financially wise).
With the blossom of mobile medical apps, it's probable that some manufacturers will be in this case.
There's no occurence of the word software in the accessories guidance. But it's clear that it is consistent in its intent with the three other ones.
And then?
The accessories guidance and the general wellness guidance are still drafts. The FDA will probably publish another one called Draft Guidance on Medical Device Decision Support Software. It is planned in the A-list of guidances for 2015.
This last one will also have some side effects existing software or future software in the plans of manufacturers.
All these draft guidances are in the B-List of final guidances of 2015. Meaning that the FDA's intent is to publish their final version, if FDA resources are available.
Medical software landscape is changing fast. So is the FDA's position, expressed in their guidance.
