Templates Repository for Software Development Process
I gather here all the templates I built about system and software development process. I sort them according to the main tasks found in software development process:
- System Requirements Analysis,
- System Architecture and System Interfaces Design,
- Software Requirements Analysis,
- Software Architecture and Software Interfaces Design,
- Software Detailed Design,
- Coding, coding, coding and coding,
- Unit Tests and Software Components Tests,
- Software Integration and Tests,
- System Integration and Tests,
- Delivery,
- System Validation.
Update july 2015: I added at the end of this page the templates for validation of software QMS tools and software in production equipment.
Templates for software development process
Phases
Phases are reduced to their minimum : system and software are designed all-in-one. Architecture, interface and detailed design are merged in one phase. Tests are reduced to one phase as well.
- Specifications (System and software Requirements Analysis),
- Design (Architecture, Interfaces and Detailed Design),
- Coding, coding, coding and coding,
- Tests (Unit tests, integration tests, verification tests),
- Delivery,
- Validation.
Management
The project is simple enough to keep everything about project management and support activities in a single document. The Project Management Plan is the central document to describe management and support activities, especially documentation and software configuration. The Risk Management Plan is still alive, it contains the risk managment activities. Don't skip it, it's an important proof that you adhere to ISO 14971. The Software Development Tool Validation Plan is an optional plan, which contains elements to help you validating software development tools, when necessary.
Specifications
The system and software specifications are merged in one phase. The Software Requirements Specifications is the central document of this phase and is filled with the results of Usability specification and Risks analysis. The Software Test Plan is the unique test plan and is derived from the requirements of the software requirements specifications.
Design
The architecture design and detailed design are merged in one phase.
The Software Detailed Design is the central document of this phase. It contains the architecture definition, the interfaces definition and the components description.
If architecture is a real concern for your project (for example, it mitigates risks), you may describe the architecture in the System Architecture Document.
Coding and Unit Tests
No changes here, If you are able to reduce the coding phase, call me, Houdini!
Tests
The software tests and system tests are reduced in one phase The Software Tests Plan is the central document of this phase and verifies the content of the software requirements specifications. The tests results are gathered in the Software Tests Report.
Delivery
Only one version delivery description for the whole system/software. And a User Manual with chapters about installation and maintenance The Version Delivery Description and the User Manual (or Instructions For Use) are the central documents of this phase.
Templates
Templates for a simple project are:
- Project Management Plan
- Risk Management Plan
- Software Requirement Specification,
- Usability Engineering File
- Usability Summative Evaluation
- Risk Analysis Report
- Software System Architecture template,
- Software Design Specification template.
- Software Test Plan template
- Software Test Description template
- Software Test Report template
- Version Description template
- User Guide template
- Verification Validation Transfer Review
You may extend the Project Management Plan with:
- Software Development Plan,
- Software Configuration Management Plan, and
- Software Development Tool Validation Plan.
Add to that management of cybersecurity:
All in one template - more simple
If your software is very small and has a very low level of risk then, I suggest you to document it in a single document.
Some reviewers may not appreciate it. So use it if you know what you are doing and if:
- It has the lowest risk (eg Class I for FDA, CE Mark or Canada, Class A for IEC 62304),
- It has only one or two use cases / functionalities,
- The length of software development (not the coding phase but the whole project from lauch to delivery) is less than 6 months,
- The size of the software team is 2 people maximum.
Templates
Translations:
Thanks to the contribution of Valentin Valchev, we have now the All-in-one template translated to Bulgarian:
Thanks to the contribution of Pablo Ojeda, we have now the All-in-one template translated to Spanish:
I invite you to translate the all-in-one template to your mother tongue (it's the most downloaded template), I'll add your contribution here.
Explanations on the standard development process
What I mean by standard development process is the one you find the most in the all the literature about software in medical devices. In IEC standards or FDA guidelines, for example. It is also named waterfall process. The templates below fit best this process. But they may be used for more recent (or sexy) processes like agile methods.
Here are the principles or logics inside these templates.
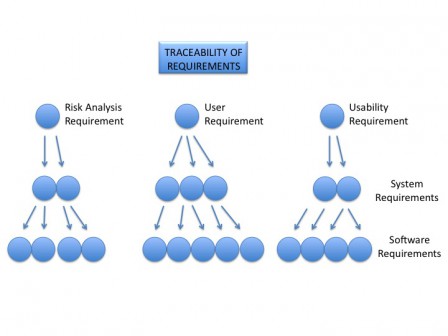
Principle of traceability
The main principle in all these templates is the traceability of data between each document. From the topmost level data: user requirements to the bottom data: conception units and tests. Most of documents have at the end a traceability table. These tables are mandatory and you shall fill them to build the tracebility tree of your software.
Here is an example of traceability tree.
Principle of verification of everything by tests
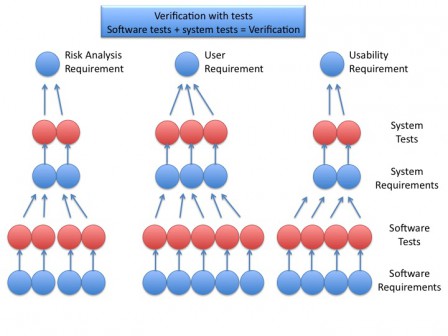
The software development is driven in parallel with the risk analysis and, if necessary, the human factors engineering. Risk and humans factors analysis give mitigations actions, which are inputs to software development. These inputs are traced during all the software development up to the software tests, which are at the end of the traceability tree. Having all tests passed allow to assert that every input, from user requirements, risk analysis and humans factory engineering, are verified.
Software tests and system tests allow to verify the requirements and, by transitivity, the whole system, as shown in the figure below.
Principle of validation through traceability
You make the validation easier if you defined well the traceability of every input and if you wrote all the documentation during the software development proccess. Hence, you will have all the proofs to show that user requirements, risks and human factors where well implemented in your software. Of course, the clinical aspects of validation remains outside the scope of the software development process. This is he job of clinicians, not software developers!
Software tools validation
Update of july 2015.
Software tools used in equipment or in QMS need validation as per 21 CFR 820.70 (i), and ISO 13485:2016 §7.5.2 and §4.1.6.
These templates are useful for the validation of software tools used in equipment or in QMS:
- The Validation Master Plan template itself, it contains general provisions for software validation,
- The Validation Protocol template, it contains the application of the VMP for a given system,
- The Validation Report template, it contains results of the validation protocol for a system,
- The Final Validation Report, it contains the conclusion of the validation of a system.
Legacy templates
Some templates have been updated with changes to regulations, standards and state-of-the-art. Older versions are present here:
- 2012 Project Management Plan
- 2012 Software Development Plan,
- 2013 Software Detailed Design
- 2018 System Architecture Document
- 2023 Software Requirements Specifications
- 2013 Software Test Plan
- 2013 Software Test Description
- 2013 Software Test Report
- 2012 Version Description Document
- 2012 User Guide
- Usability Specifications Document
- Usability Engineering File
- 2023 Software Configuration Management Plan
Published on Wednesday, 18 January 2012 by Mitch
