MD and IVD standards: IEC 60601-1 and IEC 61010-1, versus IEC 62304 - Part 2
By Mitch on Friday, 12 April 2013, 20:34 - Standards - Permalink
In the previous post, we've seen when it's mandatory to be compliant both with IEC 60601-1 and IEC 62304, and when IEC 60601-1 alone is enough.
But some manufacturers don't apply IEC 60601-1, mainly because their devices are not in contact with the patient or cannot be qualified are medical devices. We find in these categories in-vitro diagnosis instruments and laboratory instruments.
These instruments usually fall in the scope of IEC 61010-1. Let's see now the relationship between IEC 61010-1 and IEC 62304.
New Risks Section
IEC 61010-1 3rd edition was published in 2010. We're still in the transition between the 2nd and the 3rd edition. IEC 61010-1 3rd edition will become mandatory in Europe by october 2013.
A new section was added to the 3rd edition: Section 17 Risk Assessment, along with the informative Annex H. No risk management method is mandatory to address risks, but ISO 14971 is quoted in Annex H.
That makes the IEC 61010-1 closer to what medical devices manufacturers know. While ISO 14971 is mandatory for manufacturers of IVD instruments, it is fairly likely that manufacturers of laboratory instruments for medical purpose address risks with ISO 14971.
But that doesn't tell how to manage software.
When software appears in risks
Section 16 of the standard, titled Hazards resulting from application, requires to manage risks arising from software-based controls or ergonomics. And Section 17 requires to manage any other risks not addressed in the rest of the standard (including software, by extension). Here we are!
What is the standard that requires to manage software risks with ISO 14971, and contains other specific requirements to manage risks of software fro medical purpose?
IEC 62304, of course!
So, section 16 and 17 of IEC 61010-1 3rd edition advocate for IEC 62304. Yet, it is applied on a voluntary basis by manufacturers which instruments are for medical purpose, other than MD and IVD.
The diagram below represents the relationships between IEC 61010-1 and IEC 62304, inspired form the diagram shown in the previous post.
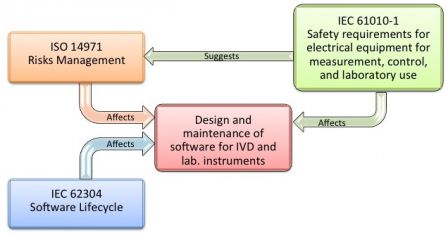
On the IEC 62304 side
IEC 62304 is more straightforward about IEC 61010-1. In Annex C, the sub-clause C.5 makes it clear about the relationship between both standards.
It states that if IVD instruments contain software that can lead to a HAZARD, then IEC 62304 must be taken into account. A flowchart is given that helps manufacturers deciding wether IEC 62304 is required or not.
Once again, this is an informative section of IEC 62304. Thus, it is applied by IVD and laboratory instruments manufacturers on a voluntary basis. It provides "food for thought", at the very least.
Conclusion
The relationship between IEC 60601-1 and IEC 62304, on the one hand, and IEC 61010-1 and IEC 62304, on the other hand, is not based on the same criteria:
- Hazards arising from software is the most important criteria to apply IEC 62304,
- IEC 60601-1 adds the notion of software complexity in its informative section:
- If software is very simple, then IEC 62304 is not necessary,
- If software is complex enough to design a software architecture, IEC 62304 becomes mandatory,
- IEC 61010-1 requires a risk assessment process with:
- Specific software hazards in Section 16,
- General-purpose risk assessment in Section 17 (including software),
- Thus making IEC 62304 relevant for instruments with a medical purpose.
