Software release vs design transfer
By Mitch on Friday, 15 May 2020, 14:00 - Processes - Permalink
A recurring question is the confusion, or more precisely the difference between software release of IEC 62304, and design transfer of ISO 13485.
A short answer is that design transfer happens after a release, but releases don't need to be attached to design transfer.
When a software development team releases a release candidate (RC) version or an alpha or a beta version (choose your nomenclature, I prefer RC), we're still in design. The product isn't validated yet.
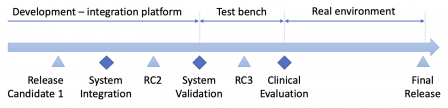
This is quite straightforward for embedded systems: the steps can be easily separated. The software is delivered to the system team for system-level tasks like integration or validation. During these tasks, additional releases may be delivered by the software team, to fix integration or functional bugs. Likewise, releases may be delivered during clinical investigations, with a very constrained change protocol, though.
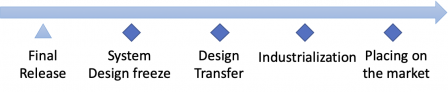
After the final release, the design is frozen and is transferred to industrialization. When industrialization processes are validated and the product is cleared by regulatory authorities, the product is placed on the market.
For software as a medical device (SaMD), releases are prone to be more frequent, as there is not a hardware specific to the device. Since there is no industrialisation step of a prototype, like methods for machining, the release and the design transfer can be merged into a single step.
Thus, the final software release and the design transfer can be performed at once during a single review.
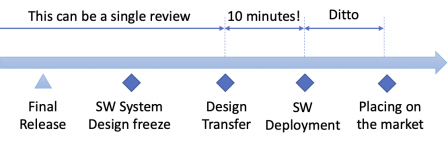
Likewise, the software deployment before placing it on the market can be a very short task, like uploading the installation bundle on the public server.
However, this is possible for small scale software system. For large-scale software system, the hardware system level can be replaced by a software system level, made of subsystems. Multiple releases of subsystems can happen before the final integration and validation of the whole system. For example, a subcontractor can deliver the release of their subsystem, before the integration and final release of the whole system.
The final release is dissociated from the design transfer, usually to complete the software documentation, like IFU's and supporting documents.
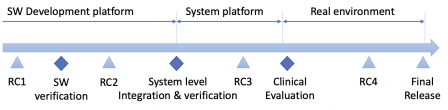
Another case where the design transfer can be dissociated from a release for SaMD is when that software is installed in the cloud. The SaMD can be released and tested on a pre-production or staging platform. Then the design transfer happens when the system is deployed to the production platform. (Note that when the software is in production in the cloud, is has to be validated according to 4.1.6 of ISO 13485).
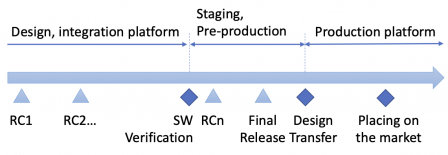
To sum up, it's possible to merge final release with design transfer for small scale projects. For large scale projects, intermediate tasks require to dissociate the final release from the design transfer.

Comments
Hi Mitch
Thankyou for your articles. I do have two questions.
1) 4.1.6 of ISO 13485 :
Did not understand why 4.1.6 is required for SaMD in SaaS context.
4.1.6 is about the validation of tools used in the development (non product software ?)
2) What would be different in the agile software development cycle for SaaS. Is there any difference at all? IEC 62304, IEC 82304 all are applicable i guess. Only the delivery/distribution is different ?
Hi Matt
IEC 62304, IEC 82304 all are applicable. I totally agree with you.
As you say, delivery is different. And this is exactly where 4.1.6 or, more precisely 7.5.6 is applicable. When the manufacturer puts the device into production on servers under their responsiblities, they shall verify that the installation went well. Thus something like an installation qualification is required.
More, if manufacturer's personnel perform some service with the SaMD (e.g. medical images segmentation / analysis) then they shall be trained to the device, and some performance qualification shall be performed.
Regards.