Hey, European Commission, it's time to copy-paste Australian regulation!
By Mitch on Thursday, 4 March 2021, 17:50 - Regulations - Permalink
The Australian Regulatory changes for software based medical devices took effect from 25th February 2021.
In the associated guidance, we find this first diagram for diagnosis:
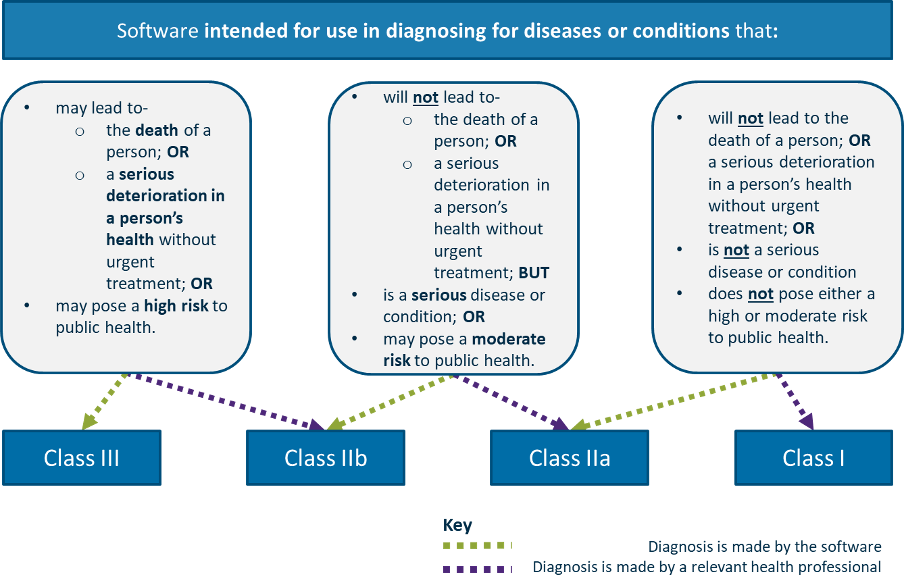
And this second diagram for treatment:
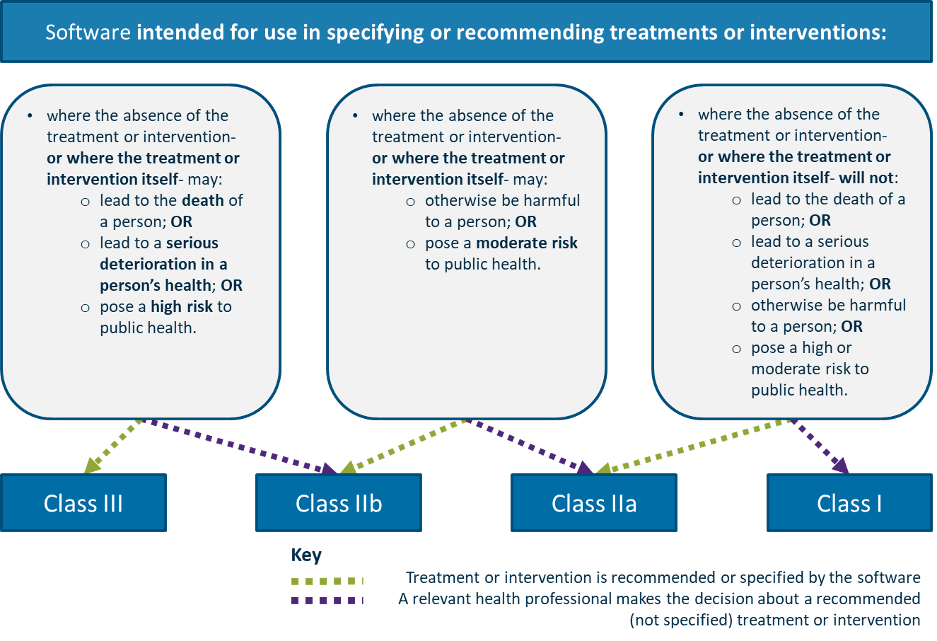
It's near perfection :-)) I have nothing to add.
Smash your rule 11 up!
Edit 2021 March 25:
To put the nail in: the TGA was updated to place some software categories outside medical devices. This came into force in January 2021, see there Therapeutic Goods (Excluded Goods) Determination 2018 the sections 14A to 14O.
In these sections, we find section 14I, excluding:
software that is:
(a) intended by its manufacturer to be used for the sole purpose of providing alerts to health professionals in relation to patient care; and
(b) not intended by its manufacturer to:
(i) replace the clinical judgement of a health professional; or
(ii) diagnose, screen for, prevent, alleviate, treat, or make a decision about the treatment of, a disease, condition, ailment or defect.
Something providing an alert that sounds pretty like a medical device with the EU regulations.
