Rest in peace class I SaMD
By Mitch on Wednesday, 26 May 2021, 00:01 - Regulations - Permalink
This is the end of MDD, and class I SaMD.
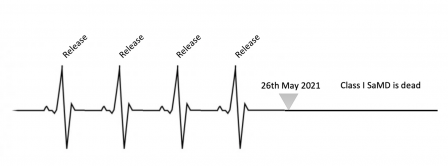
Now, it's time to stop releasing new versions with new features. This is not allowed by the MDR and confirmed by the Chart C in MDCG 2020-3.
You can only release patches and bug fixes during the grace period. Unless you CE mark the new release with the MDR, in class IIa or higher.
Anyway, this is the end of a long journey. You can congratulate your colleagues, your team, and yourself for all the tremendous work done to be on time to release and put in production the last MDD version of your software! You had hard times but you managed to do it!
If you still have bits of documentation to polish, you can do it. Continuous improvement of documentation is possible, after the 26th May 2021. Especially, if your Post-Market-Surveillance Plans is not totally well aligned with the MDR requirements, you have the right to update it afterwards. Likewise, if your risk assessment report is, say, a bit weak, you have the right to update it with post-production information.
Software design is frozen but documentation still has to follow the post-market lifecycle.
SaMD, Life, the Universe, Everything goes on!






And for French people:

The only advantage of this regulation: the vanishing of CE marks on fake medicine devices.
