MDR: Les Shadoks and MHRA: The Gibis
By Mitch on Monday, 27 June 2022, 14:50 - Regulations - Permalink
The UK Government response to consultation on the future regulation of medical devices in the United Kingdom has been published on Sunday 26th June 2022.
Note: You can't catch the cartoon reference in the title if you didn't live in France or the UK in the '70s.
The results of this consultation are full of common sense. We can see the influence of the MDR requirements in the answers. The right part of the MDR requirements, not the wrong-one. The general public who answered to this consultation already knows what's right or wrong in the MDR!
Software as Medical Device (SaMD) is covered in chapter 10.
The main takeaways are:
- Manage distant sales, aka software available on some UK cloud server, a bit like MDR article 6,
- Use IMDRF's Possible Framework for Risk Categorization and Corresponding Considerations to amend the classification rules for SaMD,
- Introduce an airlock classification rule when the software risk profile is unclear,
- Add cybersecurity and data privacy to essential requirements, like MDR GSPR 17,
- Add guidance on validation of Artificial Intelligence medical devices. It should follow the principles of IMDRF’s Software as a Medical Device (SaMD): Clinical Evaluation document.
Using IMDRF's risk categorisation, aligned with international consensus, is probably the best option to avoid under- or over-classification of SaMD. The airlock rule, like FDA De Novo pathway, is another good option. Bravo MHRA and UK government, you do it right!
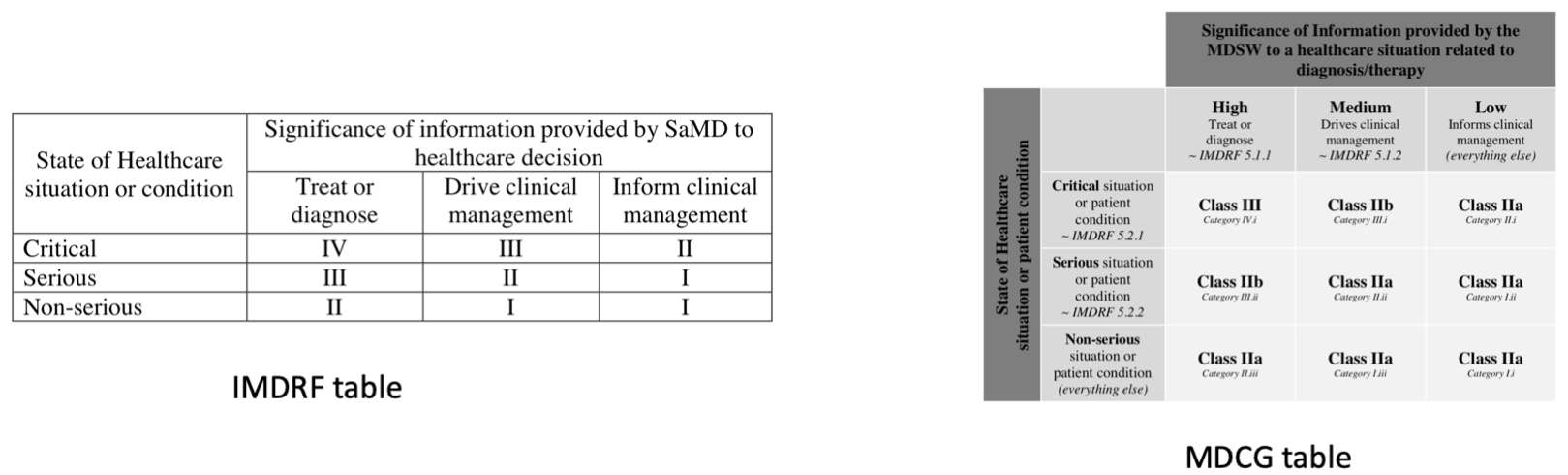
Just a little reminder: comparison between IMDRF classification on the left and the interpretation found in MDCG 2019-11 of the MDR rogue Rule 11 classification on the right:
When the Shadoks pumped, nothing happened; and the more they pumped, the more nothing happened.
