How to bring legacy software into line with IEC 62304? - part 1
By Mitch on Friday, 22 February 2013, 14:09 - Standards - Permalink
Most of medical devices manufacturers have legacy software that was not designed according to IEC 62304. The devices that embed legacy software were once verified and validated. These devices and their software work well and no major adverse event were raised by software issues.
But one day, the manufacturer decides that it's time to bring that legacy software into line with IEC 62304, to align the technical file of that software (or the contribution of software to technical file content) with up-to-date standard or regulatory requirements.
Usually, the reason that triggers this decision is either to design a new version of an existing device or to integrate that software in a new medical device.
How to do it? The content of the action plan will be different, depending on the type of legacy software or the type of changes made to the legacy software.
Types of changes made to legacy software
There are many possible types of changes that can be made to an existing software. One way to to sort them is according to the deepness of changes in design or in functionalities:
- No changes: neither architecture, nor detailed design, nor functionalities are changed. Some bugs may be fixed, though,
- Slight changes: the software architecture is not modified, only detailed design is modified, and/or existing functionalities are enhanced or updated,
- Deep changes: the software architecture is deeply modified and/or new functionalities are added.
1st case: Legacy software is not changed
This case is the most simple, and the answer is straightforward: treat legacy software as a SOUP.
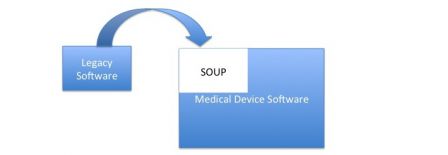
Risk analysis
The SOUP is an input data of design, first at system level and second at software level. Like any other SOUP, the necessary tasks are focussed on risk assessment:
- Identify functional and technical requirements managed by the SOUP,
- Do a risk analysis of these functional requirements,
- Do a risk analysis of SOUP integration in the medical device,
- Do a risk analysis of known bugs in the SOUP
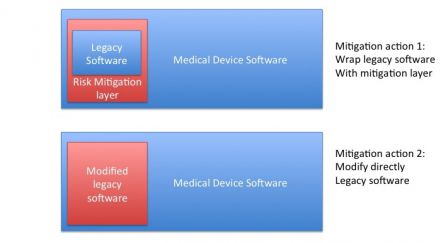
Software mitigation actions
The software mitigation actions output of the risk analysis may be of two types:
- Either the mitigation actions are made in the architecture of software that integrates the SOUP, for instance with a mitigation layer,
- Or the mitigation actions require to modify the legacy software.
The first solution is theoretically the best one with a SOUP. But the second solution may be the best to implement, when the manufacturer has the source code - and the knowledge - to modify the legacy software.
 While the second solution is probably the best, the consequence is to change the legacy software and to exit this case: modified software can't be treated as a SOUP, go to next case.
While the second solution is probably the best, the consequence is to change the legacy software and to exit this case: modified software can't be treated as a SOUP, go to next case.
Testing and mitigation proofs
Unchanged legacy software can be seen as a black box. Testing the legacy software integrated in the new device can be seen as black-box tests cases.
Verifying that legacy software runs well in its new environment and collecting the proofs of software mitigations can be made of back-box test cases. The new SW/HW components and their interfaces are tested, with legacy software integrated as a black-box in the system.
Consequence of SOUP
If the legacy software is to be used in a family of medical devices, the same problem will happen with the next version of the device or with another device in the products family. The SOUP solution is surely cost-effective at a given time, but the real solution is delayed further.
2nd case: Legacy software is slightly changed
This case is borderline, how to define slight changes?
Rule of thumb: if you have to change more that 20% of your software, redesign it!
Slight changes mean that less that 20% of software is modified. With a security margin, if only 10% of functions/components are modified or added and no architecture change is made, then this is a slight change.
If more that 10%-15% of legacy software is modified, then exit this case and go to next one.
So how to deal with this modified legacy software? Here is a solution:
- Modify it,
- Treat it as a SOUP.
Modify legacy software
If a new function is added or an existing function is modified, then:
- Do a risk analysis of the changes,
- Design the changes and mitigation actions (if any),
- Implement the changes,
- Test the modified legacy software with the changes.
The result of the risk analysis at step 1 may be to add software mitigation actions. These mitigation actions shouldn't raise the type of changes to: deep. Otherwise exit this case and go to next case.
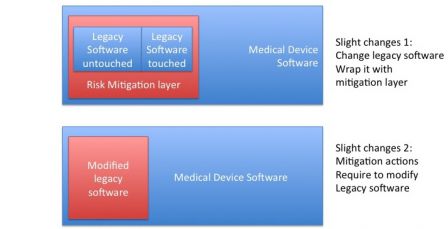
Treat legacy software as a SOUP
When changes are complete, treat the new version of the legacy software as a SOUP.
Those two phases may be planned in parallel. It's not necessary to finish the legacy software modifications to begin the design of the new device!
3rd case: Legacy software is deeply changed
Rule of thumb (bis repetita): if you have to change more that 20% of your software, redesign it!
This case arises when it's necessary to break the existing architecture or to add a big new set of functions to legacy software.
There is no miracle, the legacy software is so deeply touched that a new development cycle is compulsory. The legacy software is input data of the development cycle but it shall be redesigned along with the rest of the software it is integrated in.
Other case: Legacy software is ported to a new platform
This case is peculiar, but very common with discontinued hardware and software! The new platform may be new hardware (new microcontroller) or new software (new OS, new libraries) or both (eg: 32 bits to 64 bits). Changes made to software are:
- Technical:
- Interfaces are redesigned to work with the new platform,
- Code is fixed and recompiled to the new platform,
- Not functional:
- No existing function is modified, no new function is added,
- Existing specifications are left untouched.
This case is close to the "slight changes" case, with the major difference that the code can be deeply touched to run on the new platform.
To make it simple, it's possible to follow the same process as the one for slight changes:
- Port legacy software,
- Treat it as a SOUP.
The major difference is in the verification of the port: black-box testing is not enough. Unit testing or integration testing with modified interfaces should be necessary to demonstrate that ported software works like before.
The types of changes is not the only way to decide how to deal with legacy software. In the next post we'll see how to deal with legacy software according to their type: whether it is non-medical device software, software in a lower class or risks ... amongst others.

Comments
A great post, thanks.
Your Scenario 1 above (no changes to legacy software) seems to relate to when legacy code is integrated into a new software system, presumably one that is 62304 compliant.
How would you suggest to approach the issue of a non-compliant legacy software product which needs to comply with 62304 to remain on the market? It isn't being integrated into a new piece of software, but rather is remaining unchanged, but we need to demonstrate compliance?
Does the SOUP approach work here do you think? Could you do a development iteration as per 62304 and simply classify the software items as SOUP in the implementation step, showing suitable risk mitigation?
Your Scenario 1 above (no changes to legacy software) seems to relate to when legacy code is integrated into a new software system, presumably one that is 62304 compliant.
No, when I use the term legacy software, I mean that it was developed without any compliance to 62304. This is actually the approach that I wanted to show for a piece of legacy software integrated in a larger one. Using the SOUP approach works for legacy software that is integrated in a new development that is 62304 compliant.
How would you suggest to approach the issue of a non-compliant legacy software product which needs to comply with 62304 to remain on the market? It isn't being integrated into a new piece of software, but rather is remaining unchanged, but we need to demonstrate compliance?
Using the SOUP approach is also possible for legacy software that remains unchanged and that isn't integrated in a larger one.
Does the SOUP approach work here do you think? Could you do a development iteration as per 62304 and simply classify the software items as SOUP in the implementation step, showing suitable risk mitigation?
Your last question is not easy to answer! Two cases:
-either the legacy software is free of any risk, use it like that,
-or the legacy software bears risks. Then the development iteration is necessary to mitigate them. I would suggest to do the development with the approach of doing a wrapper around the legacy software. If your development iteration touches the source code of the legacy software, then it is no more a SOUP. And you are constrained to redo the whole software documentation compliant with IEC 62304.
Ok, so where does software that is designed, developed and tested for prototype hardware that you want to reuse fit?? Let's say we followed our QMS process to the best of our ability, but for practical reasons we did not follow all of the processes.
For instance we have designs and we have tested that software to that design. But because we lacked a released set of DIR's we don't have official artifacts to test to. i.e SRS,SAD and SDD.
We now have our 2nd phase of development starting soon which will be on production intended hardware but we want to reuse the software already designed, developed and tested, without going back to drawing board.
Classical situation, where software R&D is reused to develop the clinical version.
You have two possibilities:
1. Rely on the new requirements on legacy software added to IEC 62304 amd1 2015. These requirements are not discussed in the post, since it was written in 2013,
2. Start a new IEC 62304 compliant SW development with R&D version as input data. But reverse engineering can't be avoided.
Looks like the 2nd solution is the only one suitable for your case.
Hi Mitch,
What an interesting approach!
I assume it is relevant for any knid of SW? I mean standalone SW compliance could also be managed this way using the legacy part as a SOUP?
Thanks for the article.
Olivier
Yes, it is relevant for any kind of software, standalone or embedded.
Hi Mitch,
In your response of Wednesday 30 August 2017 you stated the possibility of using the Legacy Software requirements of IEC62304 Amd 1 2015 to bring R&D software up to the clinical version. However, how would this be allowed given that the definition of Legacy Software is "medical device software that was legally placed on the market ..."?
Hi Mitch:
Our software is developmented under IEC 62304:2006 but not IEC 62304:2006+A1:2015, is it definied as legacy sw?
Which plan do you recommend if we want to comply with the IEC 62304:2006+A1:2015?
Plan A: Do gap analysis according to the IEC 62304 clause 4.4.2-4.4.5; or
Plan B: Review our development documents and modify them if needed to comply with clause 5 to clause 9 in IEC 62304:2006+A1:2015.
In plan A, we must define the SW as legacy, right? But in plan B, we can claim the SW does not appliy to clause 4.4, right?
Thanks.