Artificial Intelligence in Medical Devices - Part 1 Introduction
By Mitch on Friday, 25 July 2025, 14:33 - Misc - Permalink
We start here a series of articles about artificial intelligence (AI) in medical devices (MD).
This series will consider mostly machine learning (ML), the most widespread type of AI. The one, which gives remarkable results, compared to classical algorithms.
Concepts
Some concepts are needed before continuing.
Artificial Intelligence in Medical Device is having AI Systems inside a medical device. The medical device can be a hardware electromedical device or a SaMD.
We see sometimes the acronym MDAI for Medical Device with Artificial Intelligence (not to be confused with AIMD - Active Implantable MD).
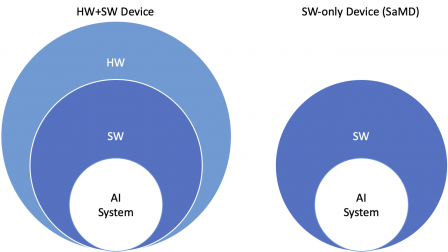
The nested items in the diagram below show that AI System is only a part of the medical device. However it may contribute significantly to the intended use of the medical device. This is more that often true for SaMD. This may be less true for electromedical devices.
Regulations
As usual, we remain focused on US and EU regulations. (even though this may be restrictive for some readers).
USA
There is no specific regulation on AI in the USA, at federal level.
The US FDA relies on the definition of device software function in the section 201(h) of the FD&C Act. This definition is quoted in the FDA guidance on Artificial Intelligence-Enabled Device Software Functions. The safety and performance of MDAI shall be validated, like any other medical device.
EU
Likewise, the EU MDR and IVDR don't have any specific requirement on AI. The safety and performance of MDAI shall be validated, like any other medical device.
We also have the AI Act. On top of the existing MDR / IVDR regulations. Bringing additional requirements to MDAI CE marking.
This is already discussed here and here.
Guidance
USA
The FDA website has a dedicated page on AI in SaMD.
Several documents have already been published. Reaching a momentum with the FDA guidance on Artificial Intelligence-Enabled Device Software Functions, already quoted above.
EU
the EU started their stream of guides, with a first MDCG 2025-6 & AIB 2025-1 document, already discussed here. It is focused on MDR/IVDR & AI Act interactions.
On a pure MDR/IVDR side, and more practical veiwpoint, the Team-NB also published a checklist on AI, already discussed here.
Other countries
We can partially complete this list with documents from other countries:
- MHRA web page on Software and artificial intelligence (AI) as a medical device,
- Health Canada Pre-market guidance for machine learning-enabled medical devices,
- TGA web page on Artificial Intelligence (AI) and medical device software.
- And the overarching IMDRF Good Machine Learning Practice for Medical Device Development.
Standards
The list of standards for MDAI is still being constructed.
We've already seen a long list of standards referenced in the Team-NB questionnaire on AI. (See here). Most of those standards aren't specific to medical devices.
Fortunately, some standards, more specific to MDAI, are being cooked by dedicated working groups. None of the standards listed below have been published yet:
- IEC 62304, of course, in its 2nd edition. It will contain new clauses on AI development plan,
- IEC 63621 on MDAI data management,
- ISO 24971-2 on risk management in MDAI, a kind of fork of AAMI 34971,
- IEC 63450 on MDAI verification,
- IEC 63521 on MDAI performance validation,
- IEC 62/540/NP on MDAI PMS (document in very early stage, no attributed standard number yet),
- And also IEC 62366-3 on MDAI usability.
We are going to see in the next articles how these future standards will shape the medical device lifecycle processes.
