Thursday, 4 March 2021
By Mitch on Thursday, 4 March 2021, 17:50 - Regulations
Continue reading...
Friday, 19 February 2021
By Mitch on Friday, 19 February 2021, 13:43 - Standards
That's a déjà-vu. The second version of IEC 62304 is still in draft. It has been in this state for five years, since the publication of the amendment 1. We already had a draft version in 2019. A new draft version is, again, in public review (or has been in public review in your country) under the name IEC CDV 62304:2021. Go to the website of your national standardization organization, to see if you can still download it for free!
Continue reading...
Friday, 5 February 2021
By Mitch on Friday, 5 February 2021, 12:40 - Regulations
A Notice was published in the Federal Register the 15th January 2021, about the exemption of a long list of medical devices from premarket notification. This list, found in table 6 of the Notice, contains 83 class II devices.
Yet, we have to wait for 120 days, to see this Notice published with the list in its final version.
Continue reading...
Friday, 25 December 2020
By Mitch on Friday, 25 December 2020, 13:55 - Regulations
After the MDCG guide 2019-11 for the MDR, the MDCG 2020-16 gives the regulatory oversight on In-Vitro Diagnostic medical devices (IVD MD) classification; including software qualified as IVD MD. Let's see the consequences for software.
Continue reading...
Friday, 20 November 2020
By Mitch on Friday, 20 November 2020, 14:01 - Regulations
The MDR 2017/745/EU and IVR 2017/746/EU define requirements, present in the Annex VII of both regulations, addressing the conformity assessment process that Notified Bodies shall put in place. It would be an understatement to say that this process will take some time.
Continue reading...
Friday, 6 November 2020
By Mitch on Friday, 6 November 2020, 12:23 - Regulations
While the winds of Covid are blowing a bad air on Europe, the UK MHRA published in September 2020 the guidance on regulatory status of medical devices, IVD and AIMD from 1 January 2021.
Continue reading...
Friday, 4 September 2020
By Mitch on Friday, 4 September 2020, 14:34 - Regulations
The FDA published in July the final version of the Guidance on Multiple Function Device Products. Despite the absence of the word "software" in the title, it addresses at first software medical devices. It also addresses hardware devices, but we will focus on software in this post.
Continue reading...
Friday, 15 May 2020
By Mitch on Friday, 15 May 2020, 14:00 - Processes
A recurring question is the confusion, or more precisely the difference between software release of IEC 62304, and design transfer of ISO 13485.
Continue reading...
Sunday, 3 May 2020
By Mitch on Sunday, 3 May 2020, 14:20 - Regulations
So we have a new guidance on cybersecurity for medical devices: the MDCG 2019-16. This is not the one we expected so quickly, but we're not going to complain about the existence of this guidance! It was published in December 2019. At last I found time to write a review.
This guidance covers a broad range of topics applicable to all stakeholders in the medical device supply chains, and to end-users. It explains a bit why it is 46 pages long.
Continue reading...
Saturday, 18 April 2020
By Mitch on Saturday, 18 April 2020, 12:03 - Misc
Having struggled with dozens of software editors who wanted to CE mark their class I software before May 2020. Having struggled with the lockdown, having struggled with projects in war mode to fight against the Covid-19, here I am! My last post was in December 2019, too long to make an active blog.
Today I post an article with a tone of speech a bit different than usual. No software, no comment on regulatory guidances. Probably an effect of the current situation, which affects all of us.
Continue reading...
Friday, 6 December 2019
By Mitch on Friday, 6 December 2019, 14:10 - Regulations
So we have a corrigendum (almost 100% sure. A vote by the EU Parliament is still in the pipe December the 16th, though). To corrigendumize: that's a neologism I propose to name bug fixing activities in legal matters. I corrigendumize, you corrigendumize, they corrigendumize! Any resemblance to "randomize" is purely coincidental!
Continue reading...
Saturday, 30 November 2019
By Mitch on Saturday, 30 November 2019, 18:20 - Templates
The final validation was missing in the list on templates on this blog. Problem solved.
Continue reading...
Friday, 22 November 2019
By Mitch on Friday, 22 November 2019, 14:16 - Standards
The second version of IEC 62304 is still in draft. It has been is this state for almost five years, since the publication of the amendment 1. It is now in public review (or has been in public review in your country) under the name IEC 62304:2019 CDV. Go to the website of your national standardization organization, to see if you can still download it for free!
Continue reading...
Saturday, 9 November 2019
By Mitch on Saturday, 9 November 2019, 20:38 - Processes
This is the Eudamed Software Development LifeCycle.
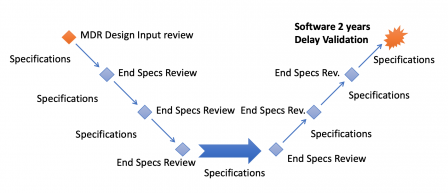
Welcome to the world of software engineering, Eudamed!
Thursday, 31 October 2019
By Mitch on Thursday, 31 October 2019, 14:03 - Regulations
Here we are! White smoke over the European Parliament! The MDCG 2019-11 guidance on qualification and classification of medical device software (MDSW) was published the 11th of October 2019.
Continue reading...
Friday, 25 October 2019
By Mitch on Friday, 25 October 2019, 21:24 - Misc
I frequently have the following discussion with software development teams: can user stories be taken as software requirements?
The answer is yes or no. All cases can be found in nature!
Continue reading...
Wednesday, 9 October 2019
By Mitch on Wednesday, 9 October 2019, 14:39 - Regulations
We know that Drug Prescription Assistance Software are software as a medical device, thanks to the European Court of Justice. But how to CE mark that kind of software?
Continue reading...
Friday, 16 August 2019
By Mitch on Friday, 16 August 2019, 13:44 - Standards
We continue this series of articles on cybersecurity with a free and non-exhaustive review of UL 2900-1 standard.
What is UL 2900-1? This standard was published in 2017 by Underwriters Laboratory (UL). It contains technical requirements on cybersecurity for network connectable products. A collateral UL 2900-2-1 focuses on connectable healthcare and wellness systems. UL 2900-1 and UL 2900-2-1 are FDA recognized standards. Thus, applicable to medical devices.
Continue reading...
Friday, 26 July 2019
By Mitch on Friday, 26 July 2019, 13:34 - Regulations
The ANSM French Competent Authority published in July 2019 a draft guideline on cybersecurity for medical devices. The European medical device sector should greatly applaud this initiative. This is the first and only guideline on cybersecurity with regard to the European medical device regulations.
Continue reading...
Friday, 19 July 2019
By Mitch on Friday, 19 July 2019, 14:10 - Regulations
The GMED notified body has published a guide on substantial changes: named Guidance document for the interpretation of significant changes in the framework of article 120: “Transitional provisions” of Regulation (EU) 2017/745. This guide addresses changes according to the article 120 of the MDR. It's a decision tree in the same vein as FDA guidances on deciding when to submit a new 510k.
As a manufacturer, your mission, if you accept it, is to answer "No" for all your products CE marked with the MDD.
For hardware, be prepared to place your design in the freezer.
For software, be prepared to place your design in liquid nitrogen.
The guidance is on the page on white papers of GMED website.

