IEC 82304-1 - latest news about the standard on Health Software
By Mitch on Friday, 15 January 2016, 14:30 - Standards - Permalink
IEC 82304-1 Health software -- Part 1: General requirements for product safety standard is still under development. Its status is visible on the page of ISO website, dedicated to IEC 82304-1. There is even a preview of the first three pages of this draft standard.
The last draft of IEC 82304-1 was published for comments in July 2015. It is a DIS (draft international standard) and should pass a step early 2016, by being accepted by the drafting committee. It means that a FDIS (final draft international standard) should be published in S1 2016. If it is accepted, the final version should be published by the end of 2016.
What is the scope of IEC 82304-1?
The scope of IEC 82304-1 intersects the scope of IEC 62304 but is not identical. It includes different types of software and different steps of the software lifecycle. IEC 82304-1 deals with health software. The definition of health software is given in the section 3.6 of the standard:
HEALTH SOFTWARE
software intended to be used specifically for maintaining or improving health of individual persons, or the delivery of care.
It is completed with the definition of health software product in the section 3.7 of the standard:
HEALTH SOFTWARE PRODUCT
combination of HEALTH SOFTWARE and ACCOMPANYING DOCUMENTS
The definition of medical device software, given at section 3.x of IEC 62304-2015 is different from the definition of health software:
MEDICAL DEVICE SOFTWARE
SOFTWARE SYSTEM that has been developed for the purpose of being incorporated into the MEDICAL DEVICE being developed or that is intended for use as a MEDICAL DEVICE
NOTE: This includes a MEDICAL DEVICE software product, which then is a MEDICAL DEVICE in its own right.
The definition of SOFTWARE PRODUCT, which was used in IEC 62304:2006, was removed from IEC 62304:2015. We now have the definition of HEALTH SOFTWARE PRODUCT in IEC 82304-1. This is one proof, amongst others, to make IEC 82304-1 and IEC 62304 a two-standard team.
Types of software
Types of software regarding the medical intended use
The first main difference between both definitions is the intended use. IEC 62304 deals only with software with medical intended use, whereas IEC 82304-1 deals with any kind of software, which directly or indirectly has an effect on health.
The scope of IEC 82304-1 is broader than the scope of IEC 62304. The following types of software are in the scope of IEC 82304-1 but not IEC 62304:
- Radiology Information Systems (RIS),
- Prescription Management Systems (PMS),
- Laboratory Information Management Systems (LIMS),
- Mobile Apps, which are not Mobile Medical Apps, according to the FDA Guidances on this subject,
- Software, which are not qualified as medical devices, according to the MEDDEV 2.1/6 EU Guidance.
Thus IEC 82304-1 includes in its scope standalone software, which are not regulated as medical devices.
Types of software regarding the platform
IEC 82304-1 deals only with standalone software. Contrary to IEC 62304, it doesn't deal with software embedded in medical devices or embedded in devices with specific hardware. Only software running on a standard PC, server, tablet, or smartphone with a general purpose Operating System are in the scope of IEC 82304-1.
The graphic below, borrowed from IEC 82304-1, shows the scope of this standard versus the scope of IEC 62304.
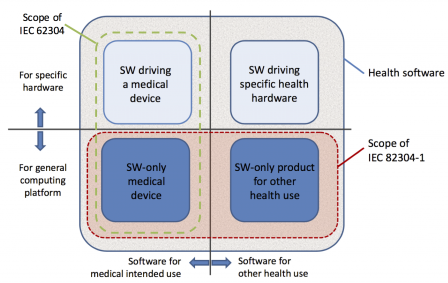 Note: the rectangle in green is not present in the graphic of the standard. It was added here for clarification to show the scope of IEC 62304.
Note: the rectangle in green is not present in the graphic of the standard. It was added here for clarification to show the scope of IEC 62304.
Steps of the software lifecycle
IEC 82304-1 deals with standalone health software product. It defines requirements at the system/product level like:
- Product Requirements,
- Product Validation,
- Product Identification and Instructions For Use,
- Post-market activities.
And it references IEC 62304:2015 for requirements at software level.
IEC 82304-1 kind of takes the place of IEC 60601-1 or IEC 61010 for standalone software. IEC 60601-1 defines requirements at system level for Programmable Electric Medical Systems (PEMS), and references IEC 62304 for the software lifecycle.
Likewise, IEC 82304-1 defines requirements at system level for health software systems, and references a subset of IEC 62304 for the software lifecycle.
The graphic below sums up this.
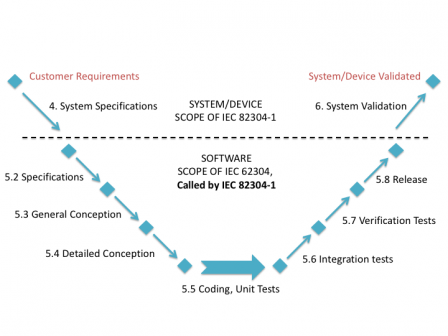 Consequence: if you want to apply IEC 82304-1 to your software, you have to apply a subset of IEC 62304 at the same time.
Consequence: if you want to apply IEC 82304-1 to your software, you have to apply a subset of IEC 62304 at the same time.
Relationships with other standards
Another way of putting this standard in the picture, it to draw the relationships of this standard with other standards, like we already did here for IEC 62304.
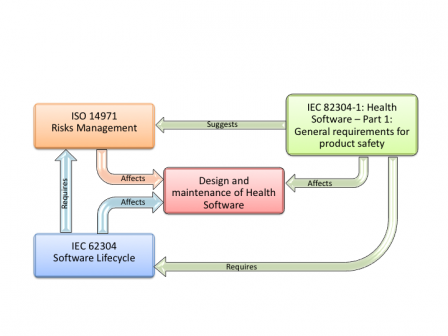 This graphic anticipates a bit what is explained in the next article: ISO 14971 is not required by IEC 82304-1 but is still required by IEC 62304.
This graphic anticipates a bit what is explained in the next article: ISO 14971 is not required by IEC 82304-1 but is still required by IEC 62304.
Is it mandatory?
Short answer:
No. A standard is never mandatory, expected in very rare cases, but surely not for health software!
Not so long answer:
Standards are never mandatory but when they are recognized by regulation authorities, like the FDA, they become "gold standards" de facto.
So, for standalone software regulated as medical devices (eg. mobile medical apps), it could become recognized by the regulations authorities as soon as the final version is published. It would make it almost mandatory.
But for standalone software NOT regulated as medical devices, since they are out of the scope of regulations authorities, the manufacturers of such software could show very little willingness to implement IEC 82304-1!
In a nutshell:
- if you develop standalone software medical devices, be prepared to see IEC 82304-1 recognized by the FDA and harmonized by the European Commission when it is published. Probably not before late 2016,
- if you develop standalone health software not qualified as medical device, we don't know what regulation authorities will make of this standard. But odds are low that it will become mandatory
Next time we'll see more in details the requirements of this standard.

Comments
Thanks for this really useful article. Just one tiny comment however, I notice there is a reference in it, to 80304 which I think should be 82304?
Hi Andrew,
Thanks for your feedback.
Yes that what a typo, fixed.